 Sometimes this orderly process breaks down, and abnormal or damaged cells grow and multiply when they shouldn’t. These cells may form tumors, which are lumps of tissue. Tumors can be cancerous or not cancerous (benign).
Sometimes this orderly process breaks down, and abnormal or damaged cells grow and multiply when they shouldn’t. These cells may form tumors, which are lumps of tissue. Tumors can be cancerous or not cancerous (benign).
Cancerous tumors spread into, or invade, nearby tissues and can travel to distant places in the body to form new tumors (a process called metastasis). Cancerous tumors may also be called malignant tumors. Many cancers form solid tumors, but cancers of the blood, such as leukemias, generally do not.
Benign tumors do not spread into, or invade, nearby tissues. When removed, benign tumors usually don’t grow back, whereas cancerous tumors sometimes do. Benign tumors can sometimes be quite large, however. Some can cause serious symptoms or be life threatening, such as benign tumors in the brain.
Differences between Cancer Cells and Normal Cells
Cancer cells differ from normal cells in many ways. For instance, cancer cells:
- grow in the absence of signals telling them to grow. Normal cells only grow when they receive such signals.
- ignore signals that normally tell cells to stop dividing or to die (a process known as programmed cell death, or apoptosis).
- invade into nearby areas and spread to other areas of the body. Normal cells stop growing when they encounter other cells, and most normal cells do not move around the body.
- tell blood vessels to grow toward tumors. These blood vessels supply tumors with oxygen and nutrients and remove waste products from tumors.
- hide from the immune system. The immune system normally eliminates damaged or abnormal cells.
- trick the immune system into helping cancer cells stay alive and grow. For instance, some cancer cells convince immune cells to protect the tumor instead of attacking it.
- accumulate multiple changes in their chromosomes, such as duplications and deletions of chromosome parts. Some cancer cells have double the normal number of chromosomes.
- rely on different kinds of nutrients than normal cells. In addition, some cancer cells make energy from nutrients in a different way than most normal cells. This lets cancer cells grow more quickly.
Many times, cancer cells rely so heavily on these abnormal behaviors that they can’t survive without them. Researchers have taken advantage of this fact, developing therapies that target the abnormal features of cancer cells. For example, some cancer therapies prevent blood vessels from growing toward tumors, essentially starving the tumor of needed nutrients.
How Does Cancer Develop?
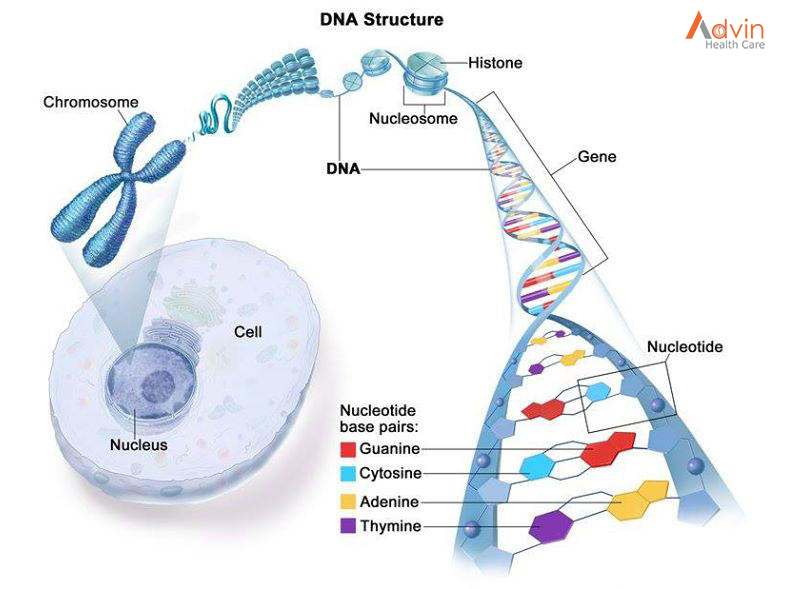
Cancer is a genetic disease—that is, it is caused by changes to genes that control the way our cells function, especially how they grow and divide.
Genetic changes that cause cancer can happen because:
- of errors that occur as cells divide.
- of damage to DNA caused by harmful substances in the environment, such as the chemicals in tobacco smoke and ultraviolet rays from the sun.
- they were inherited from our parents.
The body normally eliminates cells with damaged DNA before they turn cancerous. But the body’s ability to do so goes down as we age. This is part of the reason why there is a higher risk of cancer later in life.
Each person’s cancer has a unique combination of genetic changes. As the cancer continues to grow, additional changes will occur. Even within the same tumor, different cells may have different genetic changes.
Types of Genes that Cause Cancer
The genetic changes that contribute to cancer tend to affect three main types of genes—proto-oncogenes, tumor suppressor genes, and DNA repair genes. These changes are sometimes called “drivers” of cancer.
Proto-oncogenes are involved in normal cell growth and division. However, when these genes are altered in certain ways or are more active than normal, they may become cancer-causing genes (or oncogenes), allowing cells to grow and survive when they should not.
Tumor suppressor genes are also involved in controlling cell growth and division. Cells with certain alterations in tumor suppressor genes may divide in an uncontrolled manner.
DNA repair genes are involved in fixing damaged DNA. Cells with mutations in these genes tend to develop additional mutations in other genes and changes in their chromosomes, such as duplications and deletions of chromosome parts. Together, these mutations may cause the cells to become cancerous.
As scientists have learned more about the molecular changes that lead to cancer, they have found that certain mutations commonly occur in many types of cancer. Now there are many cancer treatments available that target gene mutations found in cancer. A few of these treatments can be used by anyone with a cancer that has the targeted mutation, no matter where the cancer started growing.
When Cancer Spreads
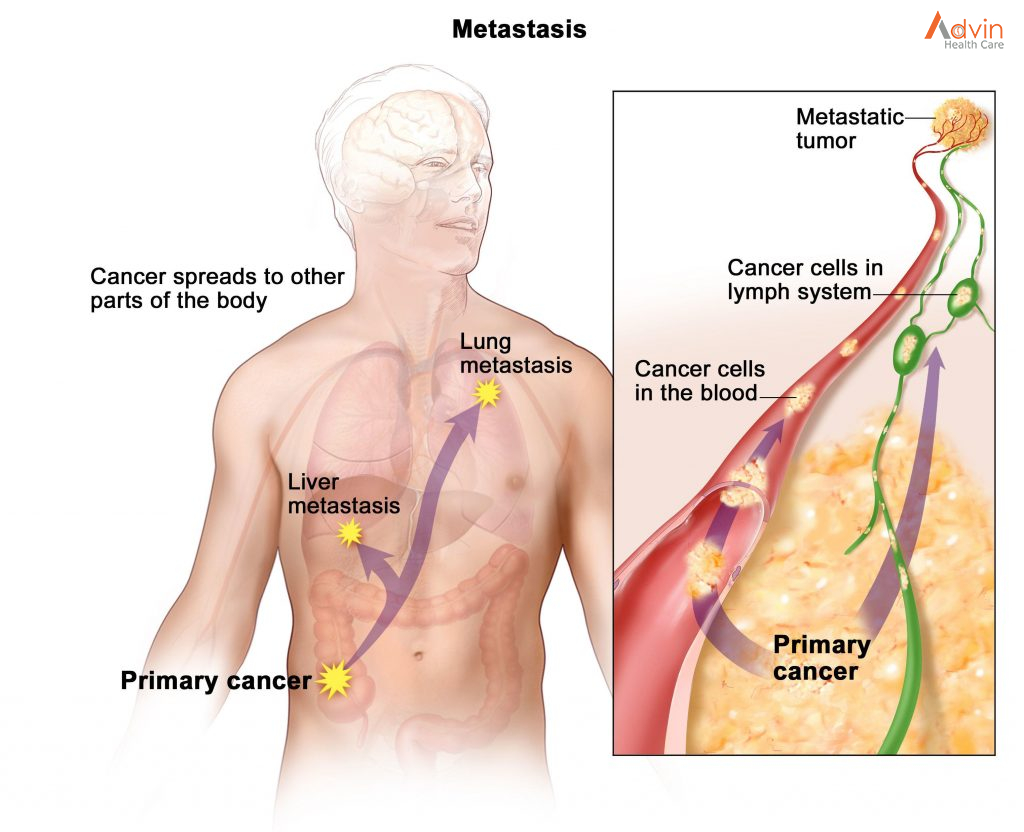
A cancer that has spread from the place where it first formed to another place in the body is called metastatic cancer. The process by which cancer cells spread to other parts of the body is called metastasis.
Metastatic cancer has the same name and the same type of cancer cells as the original, or primary, cancer. For example, breast cancer that forms a metastatic tumor in the lung is metastatic breast cancer, not lung cancer.
Under a microscope, metastatic cancer cells generally look the same as cells of the original cancer. Moreover, metastatic cancer cells and cells of the original cancer usually have some molecular features in common, such as the presence of specific chromosome changes.
In some cases, treatment may help prolong the lives of people with metastatic cancer. In other cases, the primary goal of treatment for metastatic cancer is to control the growth of the cancer or to relieve symptoms it is causing. Metastatic tumors can cause severe damage to how the body functions, and most people who die of cancer die of metastatic disease.
Tissue Changes that Are Not Cancer
Not every change in the body’s tissues is cancer. Some tissue changes may develop into cancer if they are not treated, however. Here are some examples of tissue changes that are not cancer but, in some cases, are monitored because they could become cancer:
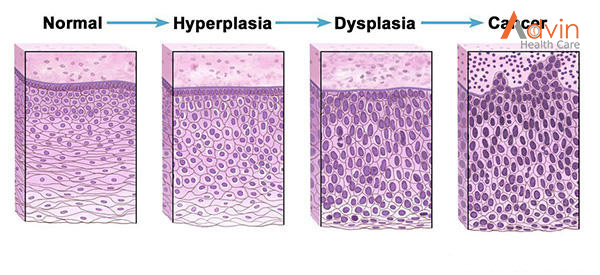
- Hyperplasia occurs when cells within a tissue multiply faster than normal and extra cells build up. However, the cells and the way the tissue is organized still look normal under a microscope. Hyperplasia can be caused by several factors or conditions, including chronic irritation.
- Dysplasia is a more advanced condition than hyperplasia. In dysplasia, there is also a buildup of extra cells. But the cells look abnormal and there are changes in how the tissue is organized. In general, the more abnormal the cells and tissue look, the greater the chance that cancer will form. Some types of dysplasia may need to be monitored or treated, but others do not. An example of dysplasia is an abnormal mole (called a dysplastic nevus) that forms on the skin. A dysplastic nevus can turn into melanoma, although most do not.
- Carcinoma in situ is an even more advanced condition. Although it is sometimes called stage 0 cancer, it is not cancer because the abnormal cells do not invade nearby tissue the way that cancer cells do. But because some carcinomas in situ may become cancer, they are usually treated.
Types of Cancer
There are more than 100 types of cancer. Types of cancer are usually named for the organs or tissues where the cancers form. For example, lung cancer starts in the lung, and brain cancer starts in the brain. Cancers also may be described by the type of cell that formed them, such as an epithelial cell or a squamous cell.
You can search NCI’s website for information on specific types of cancer based on the cancer’s location in the body
Here are some categories of cancers that begin in specific types of cells:
Carcinoma
Carcinomas are the most common type of cancer. They are formed by epithelial cells, which are the cells that cover the inside and outside surfaces of the body. There are many types of epithelial cells, which often have a column-like shape when viewed under a microscope.
Carcinomas that begin in different epithelial cell types have specific names:
Adenocarcinoma is a cancer that forms in epithelial cells that produce fluids or mucus. Tissues with this type of epithelial cell are sometimes called glandular tissues. Most cancers of the breast, colon, and prostate are adenocarcinomas.
Basal cell carcinoma is a cancer that begins in the lower or basal (base) layer of the epidermis, which is a person’s outer layer of skin.
Squamous cell carcinoma is a cancer that forms in squamous cells, which are epithelial cells that lie just beneath the outer surface of the skin. Squamous cells also line many other organs, including the stomach, intestines, lungs, bladder, and kidneys. Squamous cells look flat, like fish scales, when viewed under a microscope. Squamous cell carcinomas are sometimes called epidermoid carcinomas.
Transitional cell carcinoma is a cancer that forms in a type of epithelial tissue called transitional epithelium, or urothelium. This tissue, which is made up of many layers of epithelial cells that can get bigger and smaller, is found in the linings of the bladder, ureters, and part of the kidneys (renal pelvis), and a few other organs. Some cancers of the bladder, ureters, and kidneys are transitional cell carcinomas.
Sarcoma
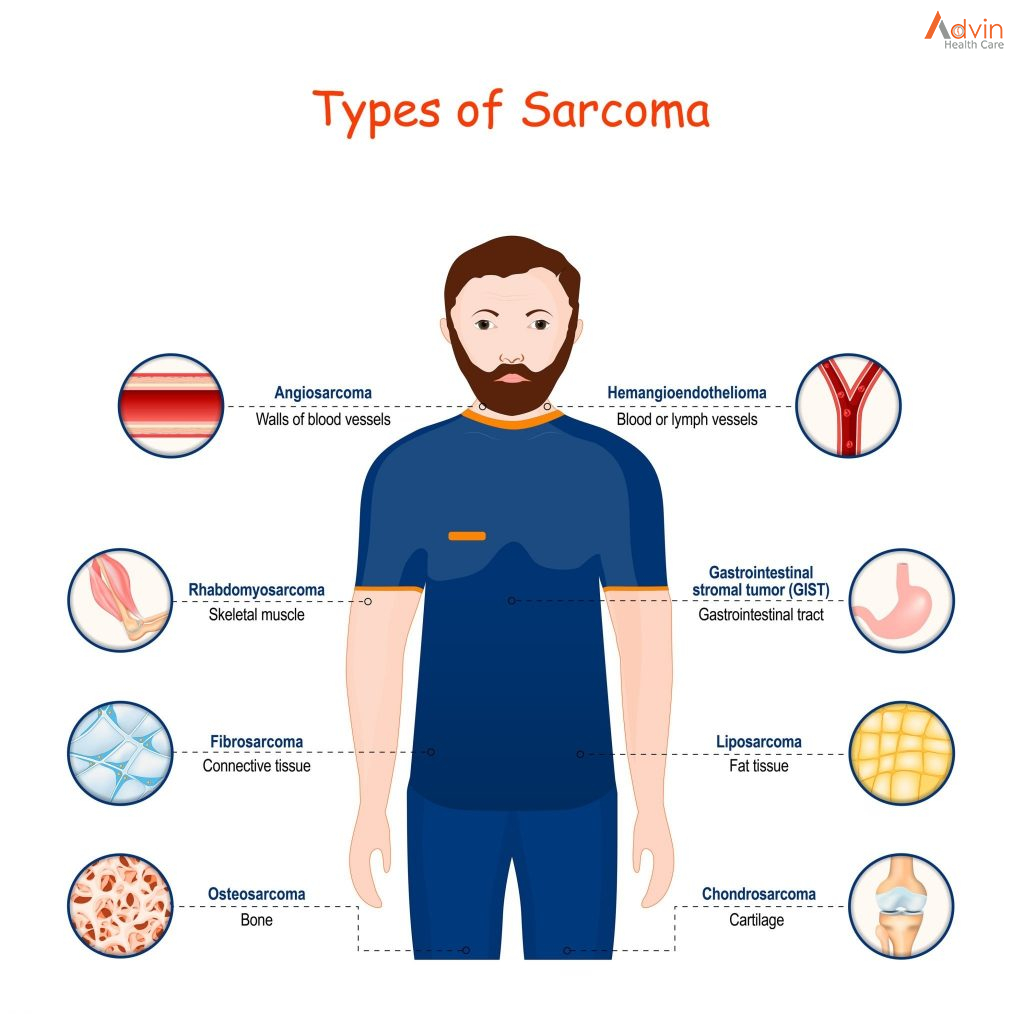
Osteosarcoma is the most common cancer of bone. The most common types of soft tissue sarcoma are leiomyosarcoma, Kaposi sarcoma, malignant fibrous histiocytoma, liposarcoma, and dermatofibrosarcoma protuberans.
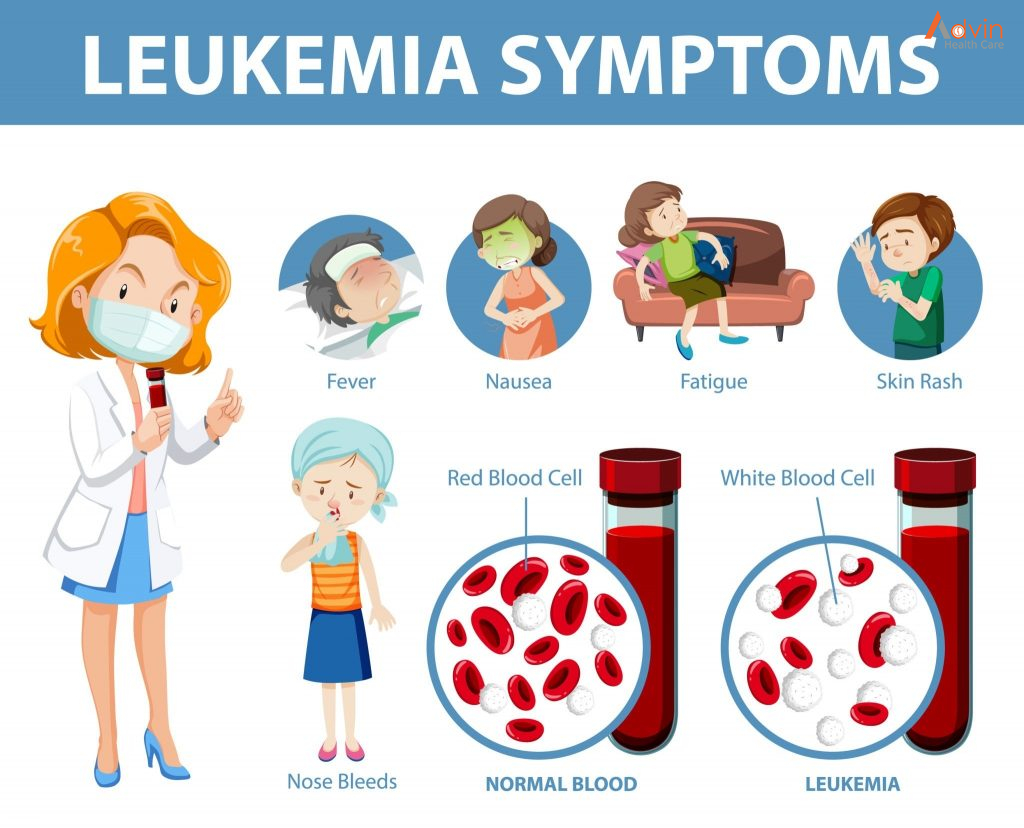
Leukemia
Cancers that begin in the blood-forming tissue of the bone marrow are called leukemias. These cancers do not form solid tumors. Instead, large numbers of abnormal white blood cells (leukemia cells and leukemic blast cells) build up in the blood and bone marrow, crowding out normal blood cells. The low level of normal blood cells can make it harder for the body to get oxygen to its tissues, control bleeding, or fight infections.
There are four common types of leukemia, which are grouped based on how quickly the disease gets worse (acute or chronic) and on the type of blood cell the cancer starts in (lymphoblastic or myeloid). Acute forms of leukemia grow quickly and chronic forms grow more slowly.
Lymphoma
Lymphoma is cancer that begins in lymphocytes (T cells or B cells). These are disease-fighting white blood cells that are part of the immune system. In lymphoma, abnormal lymphocytes build up in lymph nodes and lymph vessels, as well as in other organs of the body.
There are two main types of lymphoma:
Hodgkin lymphoma – People with this disease have abnormal lymphocytes that are called Reed-Sternberg cells. These cells usually form from B cells.
Non-Hodgkin lymphoma – This is a large group of cancers that start in lymphocytes. The cancers can grow quickly or slowly and can form from B cells or T cells.
Multiple Myeloma
Multiple myeloma is cancer that begins in plasma cells, another type of immune cell. The abnormal plasma cells, called myeloma cells, build up in the bone marrow and form tumors in bones all through the body. Multiple myeloma is also called plasma cell myeloma and Kahler disease.
Melanoma
Melanoma is cancer that begins in cells that become melanocytes, which are specialized cells that make melanin (the pigment that gives skin its color). Most melanomas form on the skin, but melanomas can also form in other pigmented tissues, such as the eye.
Brain and Spinal Cord Tumors
There are different types of brain and spinal cord tumors. These tumors are named based on the type of cell in which they formed and where the tumor first formed in the central nervous system. For example, an astrocytic tumor begins in star-shaped brain cells called astrocytes, which help keep nerve cells healthy. Brain tumors can be benign (not cancer) or malignant (cancer).
Other Types of Tumors
Germ Cell Tumors
Germ cell tumors are a type of tumor that begins in the cells that give rise to sperm or eggs. These tumors can occur almost anywhere in the body and can be either benign or malignant.
Neuroendocrine Tumors
Neuroendocrine tumors form from cells that release hormones into the blood in response to a signal from the nervous system. These tumors, which may make higher-than-normal amounts of hormones, can cause many different symptoms. Neuroendocrine tumors may be benign or malignant.
Carcinoid Tumors
Carcinoid tumors are a type of neuroendocrine tumor. They are slow-growing tumors that are usually found in the gastrointestinal system (most often in the rectum and small intestine). Carcinoid tumors may spread to the liver or other sites in the body, and they may secrete substances such as serotonin or prostaglandins, causing carcinoid syndrome.


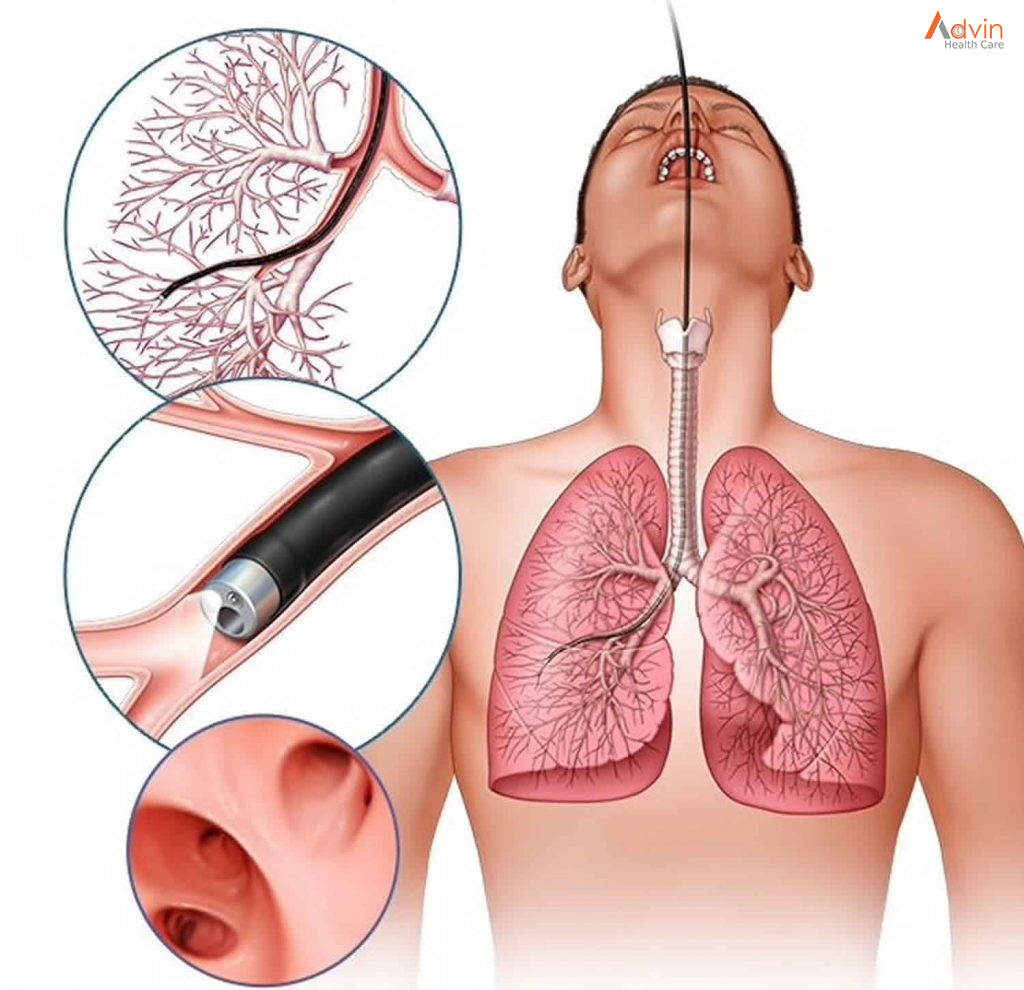










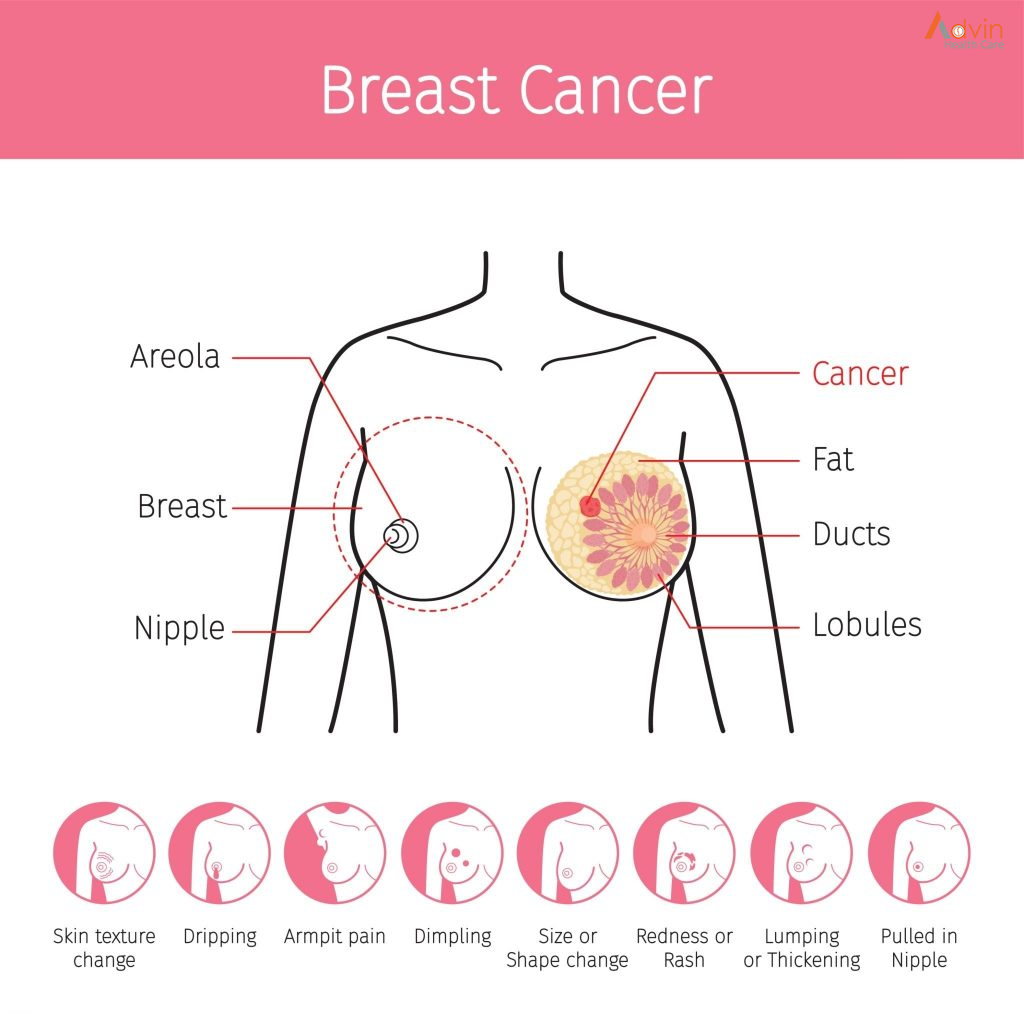 Breast cancers can start from different parts of the breast. The breast is an organ that sits on top of the upper ribs and chest muscles. There is a left and right breast and each one has mainly glands, ducts, and fatty tissue. In women, the breast makes and delivers milk to feed newborns and infants. The amount of fatty tissue in the breast determines the size of each breast.
Breast cancers can start from different parts of the breast. The breast is an organ that sits on top of the upper ribs and chest muscles. There is a left and right breast and each one has mainly glands, ducts, and fatty tissue. In women, the breast makes and delivers milk to feed newborns and infants. The amount of fatty tissue in the breast determines the size of each breast.